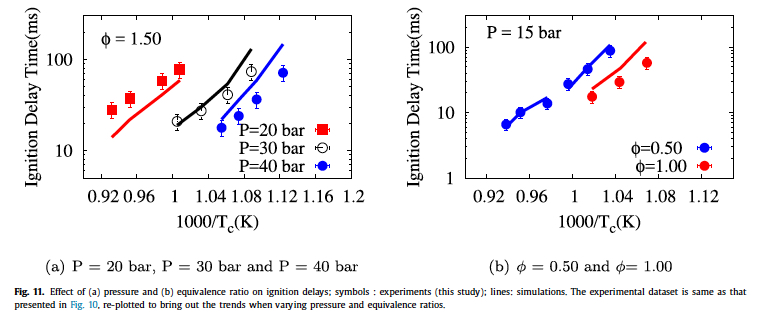
- Aditya Lele, Karan Soni, Krithika Narayanaswamy, Anand Krishnasamy, "Experimental and Modeling Investigation of NO Formation Mechanism for Biodiesel and its Blend with Methanol", WCX SAE World Congress Experience, 2019
- Shanmugasundaram Dakshnamurthy, Denis A. Knyazkov, Artem M.
Dmitriev, Oleg P. Korobeinichev, Elna J.K. Nilsson, Alexander A.
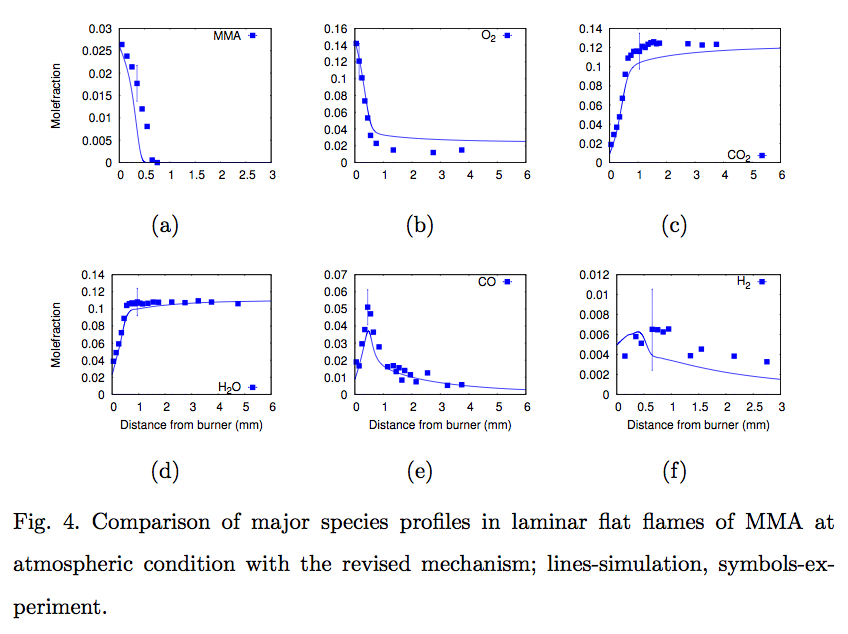
Konnov, and Krithika Narayanaswamy, "Experimental study and a short kinetic model for high temperature oxidation of methyl methacrylate", Combustion Science and Technology (in press)
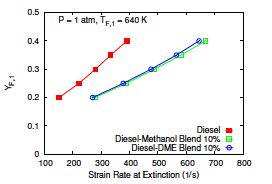
- Gerald Mairinger, Rohit Sanjay Khare, Krithika Narayanaswamy, Martin Hunyadi-Gall, Vasudevan Raghavan, Kalyanasundaram Seshadri, "Experimental and Computational Investigation of the Influence of Stoichiometric Mixture Fraction on Structure and Extinction of Laminar, Nonpremixed Methoxymethane Flames", Combustion Theory and Modelling (2018)
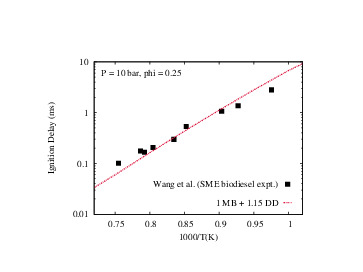
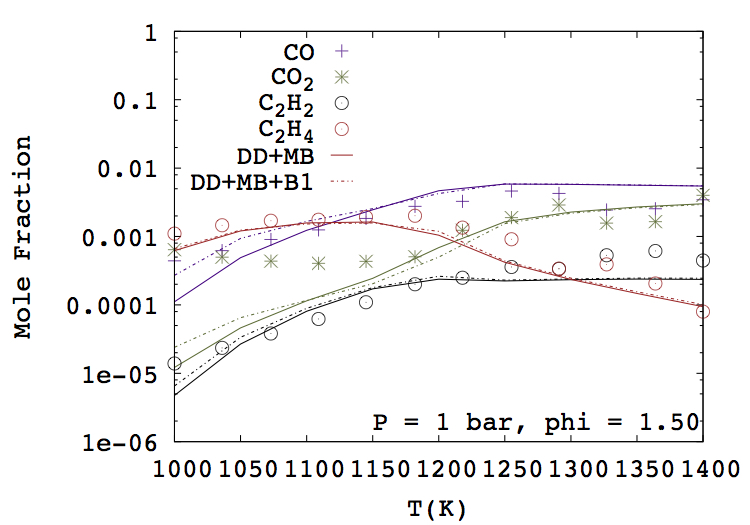
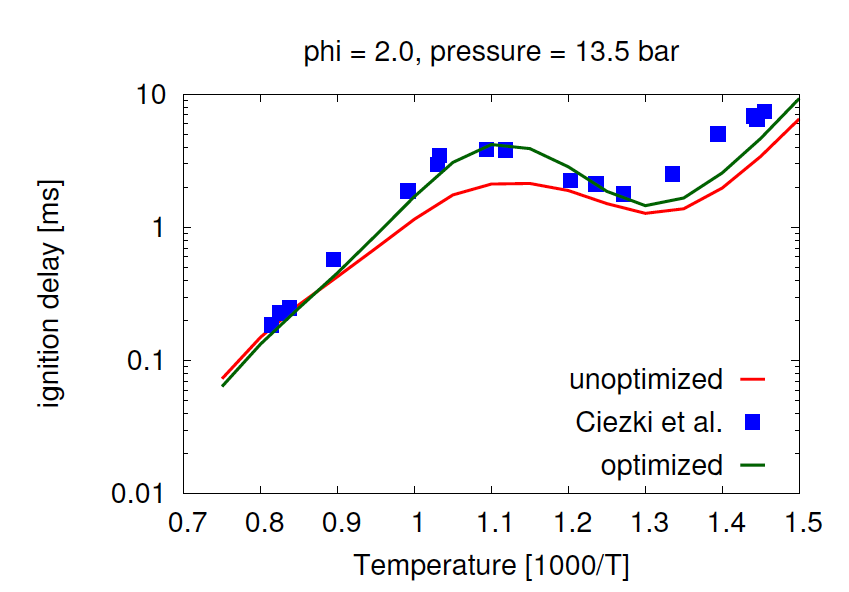
- Aditya Lele, Sonal K. Vallabhuni, Kai Moshammer, Ravi X. Fernandes, Anand Krishnasamy, Krithika Narayanaswamy, "Experimental and chemical kinetic modeling investigation of methyl butanoate as a component of biodiesel surrogate", Combustion and Flame, 197, 49-64, 2018
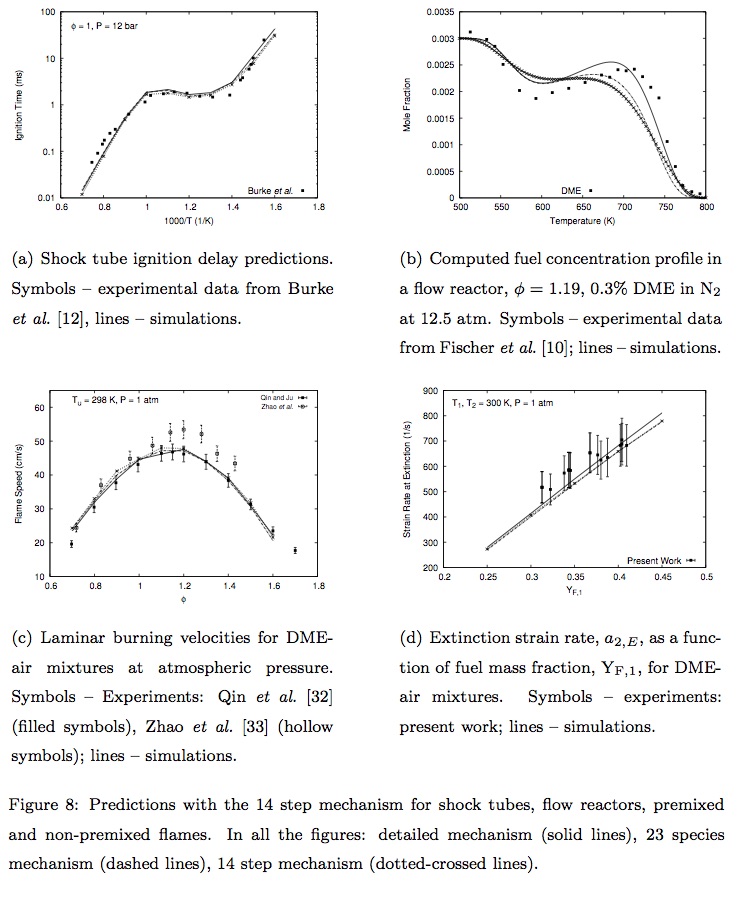
- Rohit Khare, P. Senthil Kumar, V. Raghavan, Krithika Narayanaswamy, "A comprehensively validated compact mechanism for dimethyl ether oxidation: an experimental and computational study", Combustion and Flame, 196, 116-128, 2018
- K. Narayanaswamy, P. Pepiot "Simulation-driven formulation of transportation fuel surrogates", Combustion Theory and Modelling, 1--15, 2018
- S. Ambikasaran, K. Narayanaswamy "An accurate, fast, mathematically robust, universal, non-iterative algorithm for computing multi-component diffusion velocities", Proceedings of Combustion Institute, 36 (1), 507-515, 2017.
- K. Narayanaswamy, H. Pitsch, P. Pepiot "A component library framework for deriving kinetic mechanisms for multi-component fuel surrogates: application for jet fuel surrogates", Combustion and Flame 165 (3), 288-309, 2016.
- K. Narayanaswamy, H. Pitsch, P. Pepiot "A chemical mechanism for low to high temperature oxidation of methylcyclohexane as a component of transportation fuel surrogates", Combustion and Flame, 162 (4), 2015.
- K. Narayanaswamy, P. Pepiot, H. Pitsch, "A chemical mechanism for low to high temperature oxidation of n-dodecane as a component of transportation fuel surrogates", Combustion and Flame, 161 (4), 2014.
- K. Narayanaswamy, G. Blanquart, H. Pitsch, "A consistent chemical mechanism for oxidation of substituted aromatic species", Combustion and Flame, 2010
 ORCID Page
ORCID Page